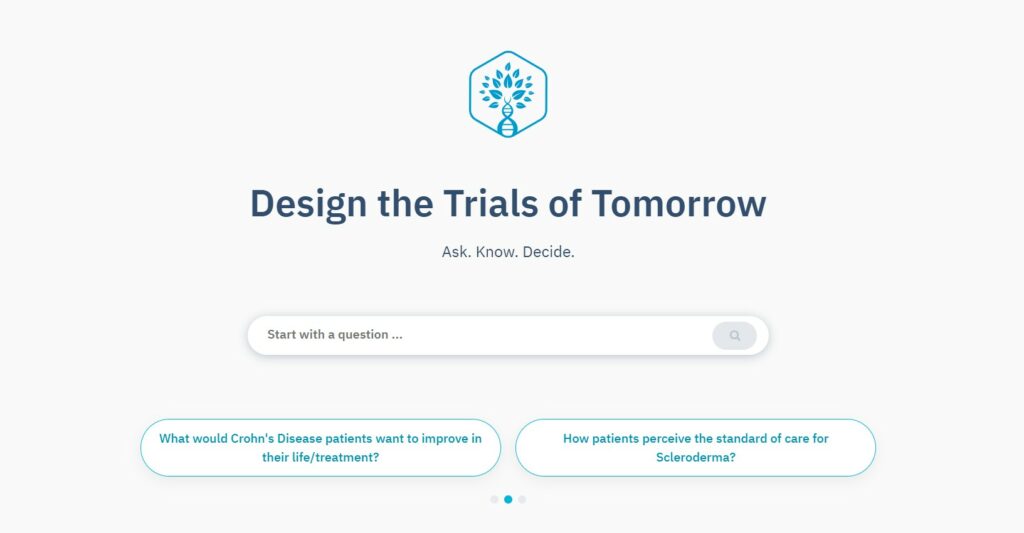
How it works
Step 1
Ask your question and watch TrialHub capture the fine details
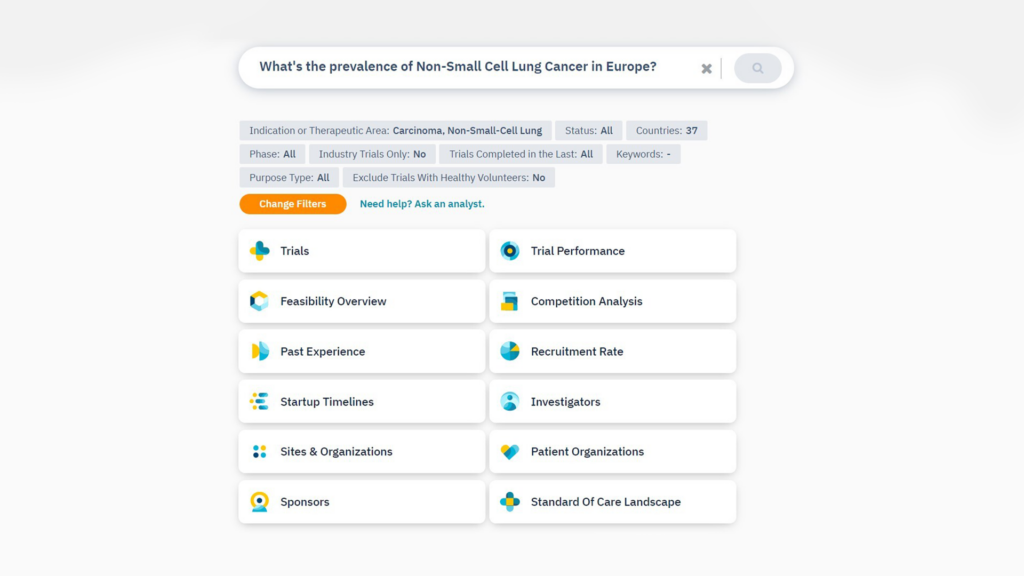
Step 2
Refine your question with additional criteria
Step 3
Jump into your detailed feasibility analysis
From Data to Insights to a Robust Strategy.
A single platform for every clinical trial expert.












on reimbursement
and Standard of Care research.
on a trial in just a quarter by avoiding substantial amendments & optimizing recruitment speed.
feasibility research and double the time to spend on strategic decisions.

on reimbursement
and Standard of Care research.

on a trial in just a quarter by avoiding substantial amendments & optimizing recruitment speed.

feasibility research and double the time to spend on strategic decisions.
smart answers
including rare diseases
data sources
database
customer care

You don’t have to waste hours of your life on tedious manual research anymore – TrialHub’s algorithms do that for you.
Our AI-driven smart answers elevate your productivity and give you more time to focus on what you do best – crafting the right strategy for your clinical trial.
You don’t have to waste hours of your life on tedious manual research anymore – TrialHub’s algorithms do that for you.
Our AI-driven smart answers elevate your productivity and give you more time to focus on what you do best – crafting the right strategy for your clinical trial.
With accurate data, gathered in real time from more than 80,000 sources and covering all indications, including rare diseases and medical devices, you’ll be sure to never miss a beat in an ever-evolving environment.

With accurate data, gathered in real time from more than 80,000 sources and covering all indications, including rare diseases and medical devices, you’ll be sure to never miss a beat in an ever-evolving environment.

Never done Patient Feasibility? Perhaps it’s been too daunting to try. Well, not anymore.
Use the industry’s most sophisticated Standard of Care data model, now upgraded with the ability to query sources like PubMed and medical guidelines.
Have your own database? TrialHub can securely connect to it and generate patient insights, uniquely available to you.

Ask your question and watch TrialHub capture the fine details
Refine your question with additional criteria
Jump into your detailed feasibility analysis
Ask your question
Refine your question with additional suggestion
Empowering the Clinical Trials of Tomorrow.
Analyze. Design. Revolutionize.